PRODUCTION
 The raw hemp material which is incorporated into Wetality products undergoes supercritical CO2 extraction in a German facility which is ISO 9001, ISO 14001 and HACCP certified.
The raw hemp material which is incorporated into Wetality products undergoes supercritical CO2 extraction in a German facility which is ISO 9001, ISO 14001 and HACCP certified.
LEGAL
For Web Page Users
 The raw hemp material which is incorporated into Wetality products undergoes supercritical CO2 extraction in a German facility which is ISO 9001, ISO 14001 and HACCP certified.
The raw hemp material which is incorporated into Wetality products undergoes supercritical CO2 extraction in a German facility which is ISO 9001, ISO 14001 and HACCP certified.
Just like GACP in agriculture, (GMP – Good Manufacturing Practices) is a golden standard of production and a norm in the pharmaceutical industry.
The requirements of GMP concern product handling, staff education, equipment, and machinery standards and cleaning, packaging, department coordination, quality assurance, etc..
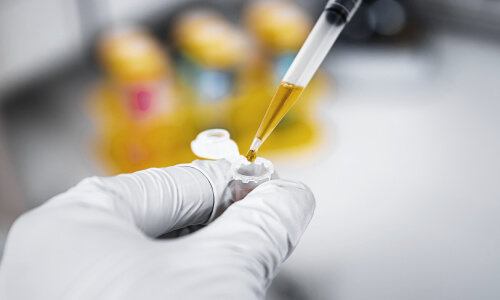
For the end-user, this guarantees that production took place in a sterile, safe environment, that the product quality is always the same, regardless of the batch and that it is essentially.
The facility in which Wetality products are manufactured
and bottled is at GMP and food-grade level with automated systems for homogenization and filling.
![]()

*free DAO or international shipping
or discounted GLS shipping (discount € 6.00)

*free DAO or international shipping
or discounted GLS shipping (discount € 6.00)